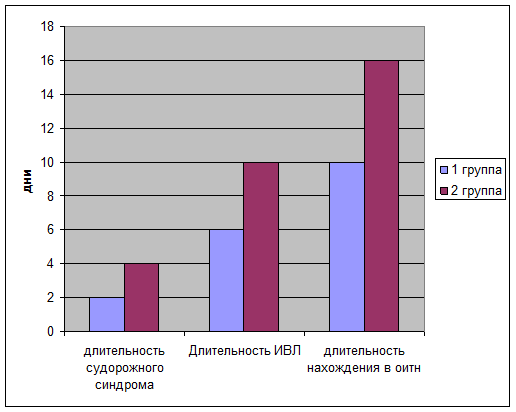
Длительность судорожного синдрома определялась ретроспективно по записям в врачебных дневниках и в первой группе составила в среднем 2-е суток, тогда как во второй группе судорожный синдром присутствовал в среднем 4 дня.
При оценке длительности нахождения на ИВЛ, выяснилось, что у детей первой группы средняя продолжительность ИВЛ составила 6 дней, тогда как у детей 2 группы эта цифра составила 10 дней. Также определено снижение общего пребывания в ОИТН детей первой группы ( в среднем 10 суток) по сравнению со второй группой (16 суток)
При проведении нейросонографии с доплерографией у детей обеих групп исследовался индекс резистентности в передней мозговой артерии на 3-и сутки жизни.
| Ri 1 | Ri 2 | Ri 3 | Ri 4 | Ri 5 | |
| 1 группа | 0.55 | 0.5 | 0.6 | 0.45 | 0.4 |
| 2 группа | 0.42 | 0.4 | 0.35 | 0.32 | 0.4 |
Среднее значение Ri в первой группе составило 0,5, а во второй- 0,38 , что свидетельствует о более неблагоприятном прогнозе у детей 2 группы.
Выводы
Выявленные нами различия у детей двух исследуемых групп с гипоксически-ишемической энцефалопатией, позволяют судить о том, что применение тотальной гипотермии у новорожденных с гипоксически-ишемической энцефалопатией:
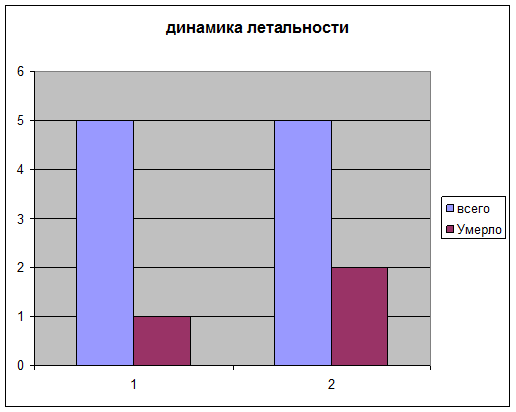
- Снижает летальность
- Снижает длительность нахождения на ИВЛ
- Снижает длительность пребывания ребёнка в отделении реанимации
- Улучшает гемодинамику в сосудах головного мозга и соответственно увеличивает шанс на благоприятный исход данного заболевания
- Укорачивает длительность судорожного синдрома
Список литературы
1. Roberston CMT, Finer NN, Grace MGA. School performance of survivors of neonatal encephalopathy associated with birth asphyxia at term. J Pediatr. 1989;114:753–760
2. Shankaran S, Woldt E, Koepke T, Bedard MP, Nandyal R. Acute neonatal morbidity and long-term central nervous system sequelae of perinatal asphyxia in term infants. Early Hum Dev. 1991;25:135–148
3. American College of Obstetricians and Gynecologist and American Academy of Pediatrics.Neonatal Encephalopathy and Cerebral Palsy. Defining the Pathogenesis and Pathophysiology. Washington, DC: 2003
4. Lorek A, Takei Y, Cady EB, et al Delayed ("secondary") cerebral energy failure after acute hypoxia-ischemia in the newborn piglet: continuous 48-hour studies by phosphorus magnetic resonance spectroscopy. Pediatr Res. 1994;36:699–706
5. Laptook AR, Corbett RJ, Arencibia-Mireles O, Ruley J. Glucose-associated alterations in ischemic brain metabolism of neonatal piglets. Stroke. 1992;23:1504–1511
6. Johnston MV, Trescher WH, Ishida A, Nakajima W. Neurobiology of hypoxic-ischemic injury in the developing brain. Pediatr Res. 2001;49:735–741
7. Siesjo BK, Bengtsson F. Calcium fluxes, calcium antagonists, and calcium-related pathology in brain ischemia, hypoglycemia, and spreading depression: a unifying hypothesis. J Cereb Blood Flow Metab. 1989;9:127–140
8. Fellman V, Raivio KO. Reperfusion injury as the mechanism of brain damage after perinatal asphyxia. Pediatr Res. 1997;41:599–606
9. Liu XH, Kwon D, Schielke GP, Yang GY, Silverstein FS, Barks JD. Mice deficient in interleukin-1 converting enzyme are resistant to neonatal hypoxic-ischemic brain damage. J Cereb Blood Flow Metab. 1999;19:1099–1108
10. Mehmet H, Yue X, Squier MV, et al Increased apoptosis in the cingulate sulcus of newborn piglets following transient hypoxia-ischaemia is related to the degree of high energy phosphate depletion during the insult. Neurosci Lett. 1994;181:121–125
11. Tan WK, Williams CE, During MJ, et al Accumulation of cytotoxins during the development of seizures and edema after hypoxic-ischemic injury in late gestation fetal sheep. Pediatr Res. 1996;39:791–797
12. Gluckman PD, Guan J, Williams C, et al Asphyxial brain injury—the role of the IGF system. Mol Cell Endocrinol. 1998;140:95–99
13. Gunn AJ, Gunn TR, de Haan HH, Williams CE, Gluckman PD. Dramatic neuronal rescue with prolonged selective head cooling after ischemia in fetal lambs. J Clin Invest. 1997;99:248–256
14. Gunn AJ, Gunn TR, Gunning MI, Williams CE, Gluckman PD. Neuroprotection with prolonged head cooling started before postischemic seizures in fetal sheep. Pediatrics. 1998;102:1098–1106
15. Gunn AJ, Bennet L, Gunning MI, Gluckman PD, Gunn TR. Cerebral hypothermia is not neuroprotective when started after postischemic seizures in fetal sheep. Pediatr Res. 1999;46:274–280
16. Sarnat HB, Sarnat MS. Neonatal encephalopathy following fetal distress. A clinical and electroencephalographic study. Arch Neurol. 1976;33:696–705
17. Spitzmiller RE, Phillips T, Meinzen-Derr J, Hoath SB. Amplitude-integrated EEG is useful in predicting neurodevelopment outcome in full term infants with hypoxic-ischemic encephalopathy: a meta-analysis. J Child Neurol. 2007;22:9:1069–1078
18. Freeman JM. The use of amplitude-integrated electroencephalography: beware of its unintended consequences. Pediatrics. 2007;119:615–617
19. Bona E, Hagberg H, Løberg EM, Bågenholm R, Thoresen M. Protective effect of moderate hypothermia after neonatal hypoxia-ischemia: short and long term outcome. Pediatr Res. 1998;43:738–745
20. Busto R, Dietrich WD, Globus MYT, Valdes I, Scheinberg P, Ginsberg MD. Small differences in intraischemic brain temperature critically determine the extent of ischemic neuronal injury. J Cereb Blood Flow Metab. 1987;7:729–738
21. Carroll M, Beek O. Protection against hippocampal CA cell loss by post-ischemic hypothermia is dependent of delay of initiation and duration. Metab Brain Dis. 1992;7:45–50
22. Colbourne F, Corbett D. Delayed and prolonged post-ischemic hypothermia is neuroprotective in the gerbil. Brain Res. 1994;656:265–272
23. O'Brien FE, Iwata O, Thornton JS, et al Delayed whole body cooling to 33 to 35C and the development of impaired energy generation consequential to transient cerebral hypoxia-ischemia in the newborn piglet. Pediatrics. 2006;117:1549–1558
24. Sirimanne ES, Blumberg RM, Bossano D, et al The effect of prolonged modification of cerebral temperature on outcome after hypoxia-ischemic brain injury in the infant rat. Pediatr Res. 1996;39:591–597
25. Thoresen M, Penrice J, Lorek A, et al Mild hypothermia following severe transient hypoxia-ischemic ameliorates delayed cerebral energy failure in the newborn piglet. Pediatr Res. 1995;5:667–670
26. Thoresen M, Bågenholm R, Løberg EM, Apricena F, Kjellmer I. Posthypoxic cooling of neonatal rats provides protection against brain injury. Arch Dis Child. 1996;74:F3–F9
27. Thoresen M, Simmonds M, Satas S, Tooley J, Silver IA. Effective selective head cooling during posthypoxic hypothermia in newborn piglets. Pediatr Res. 2001;49:594–599
28. Tooley J, Satas S, Eagle R, Silver IA, Thoresen M. Significant selective head cooling can be maintained long-term after global hypoxia ischemia in newborn piglets. Pediatrics. 2002;109:643–649
29. Tooley J, Satas S, Porter H, Silver IA, Thoresen M. Head cooling with mild systemic hypothermia in anesthetized piglets in neuroprotection. Ann Neurol. 2003;53:65–72
30. Tooley JR, Eagle RC, Satas S, Thoresen M. Significant head cooling can be achieved while maintaining normothermia in the newborn piglet. Arch Dis Child Fetal Neonatal Ed. 2005;90:F262–F266
31. Yager JY, Asselin J. Effects of mild hypothermia on cerebral energy metabolism during the evolution of hypoxic-ischemic brain damage in the immature rat. Stroke. 1996;27:919–926
32. Laptook AR, Corbett RJ, Sterett R, Garcia D, Tollefsbol G. Quantitative relationship between brain temperature and energy utilization rate measured in vivo using P and H magnetic resonance spectroscopy. Pediatr Res. 1995;38:919
33. Taylor DL, Mehmet H, Cady EB, Edwards AD. Improved neuroprotection with hypothermia delayed by 6 hours following cerebral hypoxia-ischemia in the 14-day-old rat. Pediatr Res. 2002;51:13–19
34. Covey MV, Oorschot DE. Effect of hypothermic post-treatment on hypoxic-ischemic striatal injury, and normal striatal development, in neonatal rats: a stereological study. Pediatr Res. 2007;62:646–651
35. Iwata O, Thornton JS, Sellwood MW, et al Depth of delayed cooling alters neuroprotection pattern after hypoxia-ischemia. Ann Neurol. 2005;58:75–87
36. Williams G, Dardzinski BJ, Buckalew AR, Smith MB. Modest hypothermia preserves cerebral energy metabolism during hypoxia-ischemia and correlates with brain damage: a P nuclear magnet resonance study in unanesthetized neonatal rats. Pediatr Res. 1997;42:700–708
37. Gluckman PD, Wyatt J, Azzopardi DV, et alon the behalf of the Cool Cap Study Group. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicenter randomized trial. Lancet. 2005;365:663–670
38. Eicher DJ, Wagner CL, Katikaneni LP, et al Moderate hypothermia in neonatal encephalopathy: efficacy outcomes. J Pediatr Neurol. 2005;32:11–17
39. Shankaran S, Laptook AR, Ehrenkranz RA, et aland the NICHD and Human Development Neonatal Research Network. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353:1574–1584
40. Laptook A, Tyson J, Shankaran S, et al Elevated temperature after hypoxic-ischemic encephalopathy: a risk factor for adverse outcome. Pediatrics. 2008;122:491–499
41. Wyatt JS, Gluckman PD, Liu PY, et alfor the Cool Cap Study Group. Determinants of outcomes after head cooling for neonatal encephalopathy. Pediatrics. 2007;119:912–921
42. Bramlett HM, Dietrich WD. Progressive damage after brain and spinal cord injury; pathomechanisms and treatment strategies. Prog Brain Res. 2007;161:125–141
43. Yager JY, Armstrong EA, Jaharus C, Saucier DM, Wirrell EC. Preventing hypothermia decreases brain damage following neonatal hypoxic-ischemic seizures. Brain Res. 2004;1011:48–57
44. Mishima K, Ikeda T, Yoshikawa T, et al Effects of hypothermia and hyperthermia on attention and spatial learning deficits following neonatal hypoxia-ischemic insult in rats. Behav Brain Res. 2004;151:209–217
45. Ambalavanan N, Carlo WA, Shankaran S, et alNational Institute of Child Health and Human Development Neonatal Research Network. Predicting outcomes of neonates diagnosed with hypoxemic-ischemic encephalopathy. Pediatrics. 2006;118:2084–2093
46. Oh W, Perritt R, Shankaran S, et al Association between urinary lactate to creatinine ratio and neurodevelopmental outcome in term infants with hypoxic-ischemic encephalopathy. J Pediatr. 2008;153:375–378
47. Shankaran S, Pappas A, Laptook AR, et alfor the NICHD Neonatal Research Network. Outcomes of safety and effectiveness in a multicenter randomized controlled trial of whole-body hypothermia for neonatal hypoxic-ischemic encephalopathy. Pediatrics. 2008;122:e791–e798
48. Shah PS, Ohlsson A, Perlman A. Hypothermia to treat neonatal hypoxic ischemic encephalopathy. Arch Pediatr Adolesc Med. 2007;161:951–958
49. Schulzke SM, Rao S, Patole SK. A systematic review of cooling for neuroprotection with hypoxic ischemic encephalopathy—are we there yet? BMC Pediatrics. 2007;7:1–30
50. Jacobs S, Hunt R, Tarnow-Mordi W, Inder T, Davis P. Cooling for newborns with hypoxic ischemic encephalopathy. Cochrane Database Syst Rev. 2007;4:1–46
8-09-2015, 19:10
